Electrochemically activated solutions
Electrochemical activation of solutions is always accompanied with changes of their chemical composition, acidity and alkalinity, as well as considerable increase in their reactive capacity.
The following multi-purpose solutions with high reactive capacity can be obtained from initial low-mineralized solution with less than 1 g/l mineralization:
-
Anolyte ANK SUPER possessing properties of disinfectant, sterilizing agent and detergent;
-
Katholyte К with properties of detergent and extractive agent.
What to measure to identify ECA solutions?
-
total mineralization (conductivity meter);
-
oxidant concentration (express method – test strips; more precise methods– titrimetric one by means of manual titrator or titration with a burette; photocolorimetry by photocolorimeter);
-
рН value (test strips, рН meter).
How to distinguish electrochemically activated solutions and determine a degree of electrochemical activation: the less total ion content in the activated solution, with equal content of active substances, the higher is the reactive capacity of activated solution, the longer is its lifetime, the lower is corrosive action.
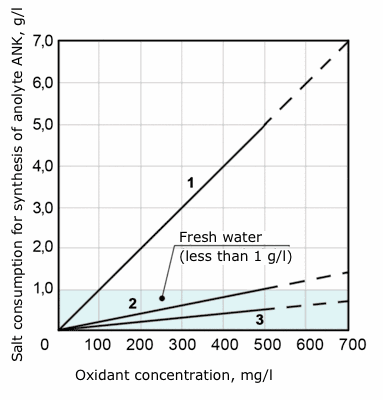
Ratio of salt used for the synthesis of 1 l of anolyte and oxidant concentration in anolyte ANK.
1 – anolyte ANK of the first generation produced by STEL-10N-120-01 devices. Salt consumption to oxidant concentration ratio: 5,0: 0,5 = 10. Ballast substances are nearly 10 times more than active substances.
2 – Anolyte ANK of the second generation produced by STEL-ANK-PRO devices. Salt consumption to oxidant concentration ratio: 1,0: 0,5 = 2. Ballast substances amount is nearly equal to active substances.
3 - Anolyte ANK of the third generation produced by STEL-ANK-SUPER devices. Salt consumption to oxidant concentration ratio: 0,5:0,5 = 1. No ballast substances.
Electrochemically activated solutions are solutions corresponding to lines 2 and 3. All mentioned devices – STEL-10N-120-01, STEL-ANK-PRO, STEL-ANK-SUPER are developments of the Institute and demonstrate stages of evolution of electrochemical devices.
Devices offered by other manufacturers on the market correspond to line 1 or generate solutions with inferior properties which currently cannot be considered to be electrochemically activated. Salt conversion rate in these devices does not exceed 10 %.
Three major components of ECA solutions – anolytes ANK of the latest generation
- Why low mineralization of ECA solutions is important:
- No corrosive action.
- No salt traces after drying out.
- Possibility to use in process cycles where the absence of ballast substances is the obligatory condition (food industry, pharmaceuticals, etc.).
- Why high oxidant concentration of ECA solutions is so important:
- Guaranteed disinfection rate of all pathogens, including spores and viruses.
- Possibility to dilute ECA solutions to extremely low concentration with disinfecting properties kept unchanged.
- Possibility to obtain non-corrosive low-mineralized ECA solutions exclusive of ballast substances with high oxidant concentration capable of disinfecting any and all pathogens in any process cycle of any industry!
- Why high salt conversion rate from initial salt solution is so important for generation of ECA solutions:
Synthesis of electrochemically activated solutions is possible only during unipolar electrochemical action in combination with processing of maximum possible amount of microvolumes of liquid in high intensity electrical field of double electric layer at the electrode surface. Specified conditions of synthesis of activated solutions can be implemented exclusively in special-purpose technical electrochemical systems.
Through long-term experience it has been found that successful ECA application critically depends on the degree of technical excellence of electrochemical devices applied and the level of maturity of technology of their use.

